Self-Propagating High Temperature Synthesis -
A Soviet Method for Producing Ceramic Materials
Joey F. Crider
U.S. Army Foreign Science and Technology Center
Charlottesville, VA 22906
Summary and Implications
Ceramic materials offer a unique combination of properties that makes them indispensable for meeting the materials requirements for many high technology applications. One of the chief military uses of ceramics is in armor systems. The combination of high hardness, high elastic modulus, and low density that some ceramics offer makes them very effective in defeating small-arms projectiles. Because their properties are retained at high temperatures, ceramics are excellent candidates for heat-engine applications.
Large-scale production of ceramic bodies by conventional powder-consolidation methods is somewhat cumbersome because high temperature furnaces (1200° to 1800°C) and relatively long processing times (several hours) are required. As a result, it is difficult to achieve and maintain high productivity. Moreover, solid-state reactions of powder mixtures in high temperature furnaces are often incomplete, allowing unreacted substances to act as impurities and leading to poor-quality products. The Soviets recognized the shortcomings of conventional ceramic-production techniques and, in the early 1970s, initiated several programs to develop better, more cost-effective production methods. These techniques included gas-phase plating, synthesis in a low temperature plasma and shock-wave compression, as well as a new method called self-propagating high temperature synthesis, or "SVS."* The SVS method is a simple, economical process discovered by the Soviets for producing high quality
ceramics and other refractory compounds through the exothermic reaction caused by the spontaneous propagation of a combustion wave through the initial reactants. This process - also called gasless combustion, high temperature chain fusion, or spin combustion - is very similar to common thermite reactions and provides the following advantages:
- The need for high temperature furnaces and complex processing equipment is eliminated;
- Large quantities of high purity ceramics can be produced rapidly and inexpensively; and
- Energy consumption is greatly reduced.
By 1976, 30 different Soviet facilities were engaged in investigating the SVS process as well as studying ceramic products made with this technique. Seventy compounds had been synthesized by 1974, and by the late 1970s, more than 200 ceramics and refractory compounds had been produced. According to the Soviets, practically all known ceramics can be produced by the SVS method. This SVS technology has been distinguished as an outstanding achievement of the Academy of Sciences of the U.S.S.R. and has been hailed as a possible replacement for conventional powder-consolidation methods for producing many refractory materials. In 1980, plans were underway to integrate SVS technology into industrial production. It now appears that MoSi2 heating elements and TiC abrasives are being produced commercially with the SVS process.
Discussion and Analysis
Introduction and Background
The SVS method for producing ceramic materials was discovered in 1967 at the Macrokinetics Lab of the Noginsk Scientific Center, a major subdivision of the Institute of Chemical Physics in Chernogolovka. A.G. Merzhanov, head of this laboratory, is credited with the discovery of SVS and has been most responsible for the promotion of this technology. I.P. Borovinskaya and V.M. Shkiro have been the other principal researchers at this institute and also took part in the discovery. Y.I. Maksimov of the Institute of Applied Mathematics and Mechanics, Tomsk State University, has also contributed greatly to the research and development (R&D) of SVS. A compendium of the major Soviet researchers and research facilities is provided in the Appendix.
The discovery of SVS was a spinoff of Soviet research that took place throughout the 1960s regarding the combustion of condensed, solid-state systems. A theory of gasless combustion had been proposed earlier after A.F. Beliaev and L.D. Komkova had observed, that the rate of combustion of thermite** appeared to be independent of pressure. In 1967, Merzhanov was studying gasless combustion and applying the classical thermal theory of combustion to thermite reactions. These investigations attempted to prove that the rate of combustion was determined solely by the reactions taking place during the decomposition stage of combustion. The decomposition of traditional thermites was too complex, however, so to simplify the model, other compositions were considered. Merzhanov and his associates chose a mixture of Ti and В for their experiments in which they hoped to obtain simple, gasless combustion. To their surprise, the product of combustion had
retained its original shape and was exceptionally hard and quite dense. Pure TiB2, commonly used as an industrial abrasive, was formed by this simple process.***
The Soviet researchers were quick to realize the potential of such a simple production process and began investigating a variety of carbides, nitrides, borides, and silicides of the transition metals (e.g. Ti, W, Zr, Nb, etc.). It was found that the initial substances could be in any physical state (e.g. gaseous, liquid, or solid). At first, SVS was considered by most of the Soviet scientific community to be merely a laboratory curiosity. It was not until the early 1970s, after a large number of refractory compounds had been synthesized, that the full potential of SVS and the impact that such a method could have on the industrial production of ceramic materials were realized.
Characteristics of SVS
The great advantage of producing ceramic materials by the SVS method is that long processing times in high temperature furnaces arc not necessary. To make TiC, for example, powders of Ti and С are mixed and press-formed into a pellet and placed in a simple, cylindrical reaction vessel, or reactor, made of stainless steel. The pellet is ignited on one end by an electrically heated coil of wire, which provides the heat impulse to initiate the chemical reaction between the Ti and С in the heated surface layer. The reaction forms a combustion wave, or synthesis wave, that rapidly spreads along the axis of the cylindrical sample forming TiC (Fig. 1). The reaction continues spontaneously and is caused by the high heat released by the combustion process. Various reactor modifications can be used to achieve high or low pressure, constant pressure, and/or cryogenic temperature operation. High pressures, for example, can be
obtained in cryogenic reactors from the evaporation of liquid N2. This type of reactor is depicted in Fig. 2 and is used for making ceramics such as TiN. Theoretically, the quantity of material that can be produced depends only on the size of the reactor. In early 1972, reactors of sufficient size were available to synthesize up to 10 kg of material per reaction.
The structure of the synthesis process is depicted in Fig. 3. As the initial substances enter the zone of heating, no chemical reactions have yet occurred. The synthesis wave then propagates to the thermal-release zone where reaction products begin to be produced. The rate of wave propagation is determined in this zone. The remaining zones complete the reaction and structurization, leading to the formation of the final product phase. Typical values of parameters that are characteristic of the SVS process are:
- Maximum temperature, 1500° to 4000°C;
- Wave-propagation rate, 0.1 to 15 cm/s;
- Thickness of synthesis zone, 0.1 to 5.0 mm;
- Rate of heating, 1000°C/s to 1 000 000°C/s;
- Intensity of initiation, 42 to 418 hW/(m·K) (10 to 100 cal/cm·s); and
- Duration of initiation, 0.05 to 4.0 s.
If the rate of wave propagation is low or if the process occurs at relatively low temperatures, the SVS process can be considered in equilibrium (Fig. 4). Under these conditions, the postprocess zones merge with the zone of heat release, forming a single transformation zone called the zone of synthesis. Synthesis, during which chemical bonds form, and structurization, during which the formation of the product phase takes place, occur simultaneously.
Depending on the maximum synthesis temperature of a given SVS system and the melting and boiling points of the corresponding reagents, the SVS process can be classified as one of three cases: 1) the gasless case, in which the materials before, during, and after synthesis are exclusively in the solid, or condensed, phase; 2) the filtration case, in which the nonmetallic gaseous reagent spontaneously enters into the reaction zone after being "filtered" through the pores of the pressed-metal-powder reagent; and 3) the condensed case, in which the reaction proceeds in the gas phase and is accompanied by condensation of the final products.
The high temperature developed during combustion results in almost complete transformation of the initial substances into the final product. Unreacted elements represent only 0.01 to 0.2 wt% of the product. High purity is therefore another advantage inherent in the SVS technique. The purity of the final product is essentially a function of the purity of the initial elements. Contamination normally does not occur. In fact, the high temperature of combustion provides a "self-purification" feature by evaporating those impurities that arc volatile and by removing oxide films on the metal particles by a reduction process. Titanium carbide formed by SVS typically will have an O content ranging between 0.02 and 0.2 wt%, and in most nitrides, the O content seldom rises beyond 0.5 wt%.
The physical structure of the final product of a SVS reaction depends primarily on the ratio of the synthesis temperature to the melting temperature of the final product. Sample dimensions are of secondary importance. Powders, sintered samples, and solidified materials from a melt have all been formed in various systems. Nonpulverized-powder products arc generally similar in geometry to the initial metal powders. Particle sizes range between 10 and 500 mm. Sintered products are in the form of cakes that can be easily machined. Products solidified from a melt enable finished articles of materials with high melting points to be produced during the course of the reaction. The Soviets believe that it may be possible to actually cast some refractory materials.
Direction of SVS Research
Following the discovery of SVS in 1967 at the Institute of Chemical Physics, Chernogolovka, researchers at that institute began to study the mechanisms of gasless combustion in more detail. The effects of combustion parameters on the formation of desired end-products were experimentally determined. After SVS became accepted by the overall scientific community as a viable materials technology, research began in earnest. In 1972, development was initiated to advance the manufacturing technology of SVS production methods. An experimental facility was built at the Institute of Chemical Physics to produce powders of various refractory compounds. Reaction vessels and ancillary equipment were custom made by researchers at that institute. This equipment subsequently was adopted by other Soviet research institutes that were also becoming involved in further developing this new technology. Research and development during the early 1970s included studies of the effects of N2 pressure on
the synthesis of nitrides, the effect of the proportion of metal powders to В powders on the composition of borides, and investigations of the optimum conditions of synthesis of high performance carbides such as TiC.
By 1976, at least 30 different organizations were engaged in SVS R&D. The major efforts were still being conducted at the Institute of Chemical Physics, Chernogolovka, as well as at the Institute of Applied Mathematics and Mechanics, Tomsk State University. Research programs were becoming more analytical and less empirical in nature. The mechanisms of heat transfer, chemical reactions, and product formation and kinetics of SVS processes were some of the topics under study at this time. The development of compounds having optimum property values (e.g. maximum hardness) were of practical interest. Both nonstoichiometric compositions and solid solutions of two or more binary compounds showed potential for providing these characteristics, although the methods for synthesizing these materials were much more difficult than those for simple binary compounds.
More than 200 different compounds had been synthesized by the late 1970s, and the Soviets believed that it was possible to produce almost all known refractory compounds. The production of even beryllides, phosphides, and rare-earth metals appeared feasible. Weakly exothermic compounds, including SiC and B4C, which are characterized by low bonding energies between the atoms, require an additional source of heat or furnace preheating to augment the heat of combustion. The Soviets have observed that it is possible to carry out a weakly exothermic reaction jointly with a strongly exothermic SVS process. This method would be economically attractive. The use of catalytic agents is also possible as a means of promoting the reaction.
In 1978, SVS technology gained state recognition and further financial support. Plans were formulated to construct a new, larger R&D facility in Chernogolovka. The Soviet State Committee for Science and Technology (GKNT) created a scientific council to address problems associated with the theory and practice of SVS processes. This council is headed by A. G. Merzhanov, the founder of this technology. Several problems identified by the council have included personnel training and the production of SVS equipment. Apparently, reactors and other equipment needed to meet the needs for high volume industrial production have not been readily available in the U.S.S.R. In 1980, the production of this equipment still remained a problem. Programs to train specialists in SVS technology have not yet been initiated at the higher technical schools. The SVS scientific council has recommended that such schools be formed at several institutes in the Moscow area, with the Chernogolovka branch of the
Institute of Chemical Physics acting as coordinator.
Results and Applications of SVS Research
By 1976, at least 50 common binary refractory compounds having stoichiometric compositions had been synthesized by strongly exothermic SVS reactions:
Carbides - TiC, ZrC, HfC, VC, NbC, Ta2C, and TaC;
Borides - TiB, TiB2, ZrB2, HfB2, VB, V3B2, VB2, NbB, NbB2, TaB, TaB2, and MoB;
Nitrides - Mg3N2, BN, A1N, SiN, TiN, ZrN, HfN, VN, NbN, Ta2N, TaN (hexagonal), and TaN (cubic);
Silicides - TiSi3, ZrSi, ZrSi2, and MoSi2;
Chalcogenides - MgS, NbSe2, TaSe2, MoS2, MoSe2, WS2, and Wse2;
Hydrides - TiH2, ZrH2, NdH2, CsH2, PrH2, and IH2; and
Aluminides - NiAl and CoAl.
At least seven nonstoichiometric materials had also been produced:
Carbides - TiC0.60 to 0.99 and ZrC0.60 to 0.99; and
Nitrides - TiN0.91 to 0.97, ZrN0.71 to 0.96, NbN0.92 to 1.02, HfN0.81 to 1.07, and TaN0.90 to 1.06.
A series of solid solutions composed of two discrete binary compounds had been produced as well, including TiC-TiN, ZrC-ZrN, NbC-NbN, TaC-TaN, TiB2-MoB2, TiB2-CrB2, ZrB2-CrB2, TiC-WC, MoS2-NbS2, WS2-NbS2, TiN-ZrN, and Zr-Nb-C-N.
Separately classified are compounds that were formed by weakly exothermic reactions:
Carbides - B4C, Al4C3, SiC, and Mo2C;
Borides - MoB4, MoB2, WB, W2B5, and WB4;
Silicides - TaSi2 and Mo3Si;
Aluminide - NbAl3; and
Germanide - NbGe2.
Unique material properties have been reported as a direct result of the SVS fabrication method. In 1969, SVS technology yielded а cubic polymorph of TaN that exhibited a microhardness of 31 400 MPa. This ceramic normally forms only the hexagonal crystal structure with a typical microhardness of 11 080 MPa. Such features inherent in SVS further enhance the usefulness of this materials technology. Other distinct materials have also been obtained as a direct result of the unique features of SVS processes. For example, SVS-produced MoS2 alloyed with Nb produced a superconductor.
In the early 1970s, after both SVS-produced TiC and titanium carbonitride had been characterized, it was envisioned that these SVS materials would be able to replace costly W-containing ceramics for machine-tool applications. The SVS technology is cost-effective, and the Soviets already had foreseen that W was becoming a scarce clement and should be conserved. This insight resulted in SVS being recognized in 1973 by the Soviet hierarchy as a notable achievement of the U.S.S.R. Academy, of Sciences. Between 1977 and 1979, the Institute of Chemical Physics, the Institute of Problems in Materials Science, and the Poltava Artificial Diamond and Diamond Tool Plant studied the effects of using SVS-produced TiC as a substitute for certain diamond products. A cost savings of 39 million rubles was reported during the test period with a savings of 29 million rubles for 1979 alone. In 1980, the program was transferred to the All-Union Production Association Soyuzabraziv of the Ministry of the
Machine Tool and Tool Industry for complete introduction into the Soviet industrial sector. A new abrasive paste, called KT, is now commercially available. The KT consists of grain-graded, SVS-produced TiC powders, a binder, and lubricants. These pastes, used primarily for polishing and honing, reportedly result in manpower savings of up to 80% and improved surface finishes; they reduce the degree to which the abrasive is rubbed into the part.
In 1980, Tomsk University researchers reported that Ti-Ni intermetallic compounds had been produced by SVS. This material exhibits a memory effect independent of the production method used, but SVS methods were more cost-effective and yielded products of higher purity, which is very important in achieving high quality Ti-Ni. Hundreds of kilograms of this material have been produced by this method. The Soviets are using the material in unspecified aircraft and putting the memory effect to advantage by shrink-fitting couplings in fuel and air lines. (Titanium-nickel has been used in the U.S. Navy's F-14 fighter aircraft for similar applications.) Soviet researchers have also reported that they have now obtained molten Ti-Ni products directly by the SVS process, allowing the material to be cast into ingots.****
Applications of SVS products noted by the Soviets include abrasives, high temperature heating elements and electrodes, solid lubricants, semiconductor materials, and polishing pastes. The SVS technology has been used to apply various protective coatings to metal substrates and in such diverse applications as the production of N and P fertilizers. Some Soviet researchers envision SVS as a possible means for actually casting high temperature refractory materials. Other potential uses that could have significant implications are the production of nonferrous metal powders, the direct reduction of Fe from ferrous ores, and the smelting of high alloy, high temperature metals.
At least two SVS materials apparently have now reached industrial production. As previously mentioned, TiC abrasives are now being used extensively at the Poltava Artificial Diamond and Diamond Tool Plant as a substitute for diamond products. Molybdenum-disilicide heating elements arc also being produced commercially as a result of joint efforts between the Armenian Institute of Chemical Physics and the Institute of Chemical Physics in Chernogolovka. The production cycle was "cut 50%" using SVS techniques. Operating characteristics of MoSi2 heating elements were improved, and service life was doubled. Successes such as these will undoubtedly promote additional industrial applications of SVS technology.
Problems Associated with SVS
Even though the outstanding characteristics of SVS have been clearly demonstrated by the Soviets, several problems limit the near-term extent to which SVS technology can be applied commercially. Industrial production of ceramic materials using SVS techniques has just begun. As previously mentioned, the scientific council that was established to address SVS problems identified a lack of SVS equipment production and a scarcity of SVS-trained personnel as two major problems. Probably the greatest problem facing the Soviets in the widespread adoption of SVS lies in their capacity for producing metal powders. By 1976, the Soviets had recognized the need for expanding metal-powder production; however, additional supplies would likely be diverted to the powder-metallurgy industry, which is receiving increased emphasis in the U.S.S.R.
Conclusions
The Soviets have demonstrated that SVS is a cost-effective method for producing a variety of ceramic materials. Some materials exhibit exceptional properties as a direct result of the uniqueness of the SVS process. This materials technology has demonstrated its potential for industrial-scale production of refractory compounds and, in the case of TiC and MoSi2, is replacing conventional ceramic-production processes, which consume large quantities of energy.
Bibliography
- G. Alova, "Born of Fire," Voenna Tekhnika, 8, 20-1 (1972).
- I. P. Bogoviskaya, "Combustion Processes and Chemical Synthesis"; p. 29 in Proceedings of the 3d International Symposium on Combustion Processes, Kazimierz, Poland, 1973.
- B. I. Ostroukhov and G. G. Bondareva, "New Materials for a New Technology - High-Melting Fusion Bypasses High-Temperature Furnaces," Soviet Export, 6 [93] 10 (1974).
- M. V. Keldysh, "1973 Achievements at the Academy of Sciences of the U.S.S.R.," Sotsialisticheskiya Industriay, 6, 5 (1974).
- A. G. Merzhanov, "Self-Propagating High-Temperature Synthesis of Refractory Compounds," Moscow Vestnik Akademii Nauk SSSR, 10, 76-84 (1976).
- Anonymous. "Self-Propagating High-Temperature Synthesis," Sotsialisticheskaya Industriya, 144 [2126] (1976).
- V. Istomin, "Self-Propagating High-Temperature Synthesis for Making Refractory Compounds," Sotsialisticheskaya Indusiriya, 50 [3241] 4 (1980).
- A. G. Merzhanov, "Method of Synthesizing Refractory Materials. Hard Alloys Merits Support." Izvestiya, 99 [19448] 2 (1980).
- L. Levitskiy, "Unique Alloys from High-Temperature Self-Propagating Synthesis," Izvestiva, 99 [19469] 3 (1980).
___________________
*The term, "SVS", is transliterated from the Cyrillic "CBC", the Soviet acronym for this process.
**Thermite is a mixture of Al powder and iron oxide which when ignited forms Al2O3 and molten Fe by a strong exothermic reaction.
***Normally, TiB2 is obtained by gradual healing of the powder mixture to 1500°C in a high temperature furnace and holding it at this temperature for several hours.
****If given the proper thermomechanical treatment, Ti-Ni can revert to a predetermined geometry with the application of heat via a martensitic phase transformation.
Appendix. Major Facilities and Personnel Involved in SVS Research
Institute of Chemical Physics, Chernogolovka
A. G. Merzhanov, discoverer and proponent of SVS and chairman of SVS Scientific Council; I. P. Borovinskaya, codiscoverer of SVS, data collection on silicides, and theoretical investigations; V. M. Shkiro, codiscoverer of SVS; E. A. BIyumberg, data collection on borides; V. K. Enman, process development; A. K. Filonenko, data collection; L. N. Gal'perin, process development; V. I. Itina, data collection; B. I. Khaykin, theoretical investigations; E. I. Maksimov, data collection; N. P. Novikov, data collection; B. V. Novozhilov, combustion-rate computations; V. A. Podergina, data collection; and V. I. Ratnikov, process development.
Institute of Applied Mathematics and Mechanics, Tomsk State University
Y. I. Maksimov, codiscoverer of SVS and data collection on TiC.
Institute of Problems in Materials Science, Kiev
A. V. Bochko and G. G. Karyuk, data collection on TiC.
Institute of Physics of Solids
K. M. Nikul'shina, V. S. Shekhtman, and G. A. Vishnyakova, analysis of SVS products.
Institute of Nuclear Physics
I. A. Karimov and V. F. Petrunin, neutron diffraction analysis.
Siberian Physical-Technical Institute, Tomsk State University
V. Itin, data collection on Ti-Ni, and V. Khachin, development of Ti-Ni aircraft fuel couplings.
Armenian Institute of Chemical Physics, Yerevan
S. K. Dolukhanyan, data collection on hydrides; A. P. Aldushin, theoretical investigations; A. G. Merzhanov, N. A. Martirosyan, and M. D. Nersesyan, data collection in hydrides; and K. G. Shkadinskiy, theoretical investigations.
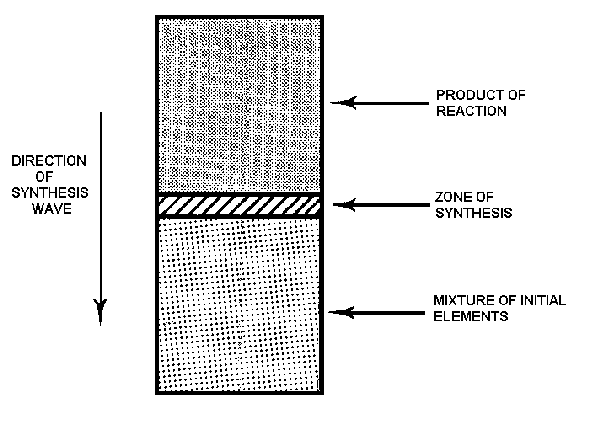
Fig.1. Diagram of the SVS process.
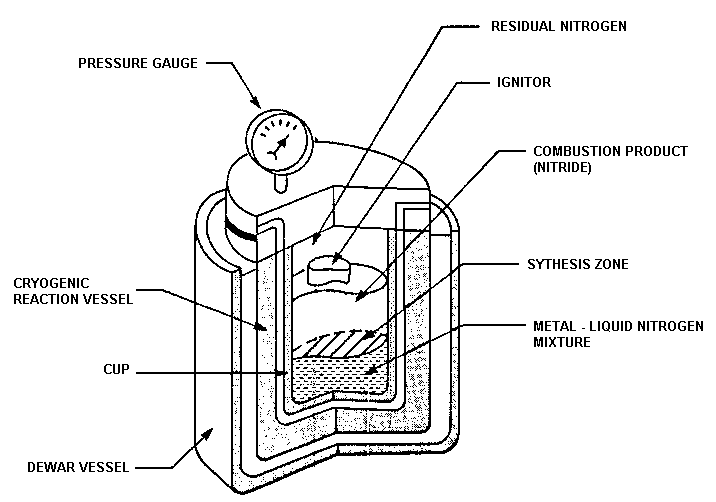
Fig.2. Cryogenic reactor for producing ceramic materials by the SVS method.
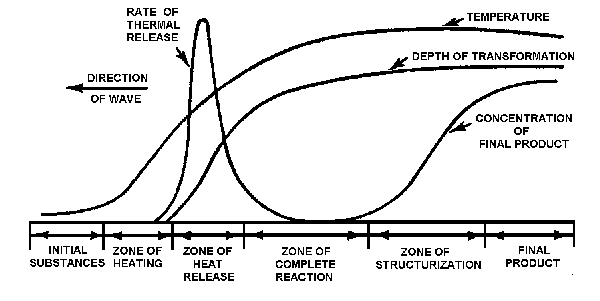
Fig.3. Nonequilibrium adiabatic structure of synthesis wave.
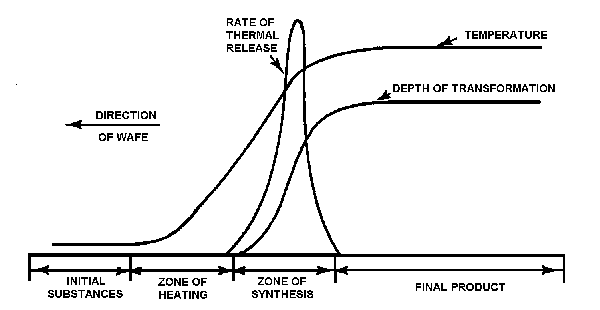
Fig.4. Equilibrium adiabatic structure of synthesis wave.



