SELF-PROPAGATED HIGH-TEMPERATURE SYNTHESIS
OF REFRACTORY INORGANIC COMPOUNDS
| A.G.Merzhanov and I.P.Borovinskaya | UDC 546 |
The synthesis of refractory inorganic compounds: carbides, nitrides, borides, sulfides, silicides, etc. usually is conducted in the condensed phase in furnaces at 1000-2000°C [1-4]. The interaction of the initial components under such conditions involves definite difficulties of a macrokinetic character, since the reagents are separated during the reaction by a film of the product, which possesses great resistance to diffusion at these temperatures. Recently the method of precipitation from the gas phase with condensation of the products on special substrates or in the volume, has achieved widespread use [5-8].
This work proposes an essentially different approach to the synthesis of refractory compounds. It is based on the use of characteristic peculiarities of the interaction of most elements of the periodic system with boron, carbon, nitrogen, silicon, etc: a) the strong exothermic effect of the process, associated with the high heat of formation of the product; b) high values of the activation energy, due to the strong dependence of the diffusion resistance of the film of the product on the temperature.
In view of this, the formation of refractory compounds in most cases can be assigned to the category of combustion reactions, which, as is well known, possess a number of noteworthy peculiarities. One of them is the possibility of occurrence of the reaction within a narrow zone, which is displaced along the substance on account of heat transfer after local initiation of the reaction in an unheated mixture of the reagents, and served as the basis for the creation of a new method for the production of refractory inorganic compounds. It was developed by the authors together with V.M. Shkiro [9] and has received the name of "self-propagating high-temperature synthesis" (SHS).
The article describes experiments on carrying out processes of SHS with the direct interaction of two chemical elements, one of which, the fuel (usually a metal), is in the condensed state, while the other, the oxidizing agent (nonmetal), is either in a condensed or in a gaseous state. Depending on the aggregated state of the oxidizing agent, three types of combustion occur in the systems: solid fuel -
solid oxidizing agent (sol+sol), solid fuel -
gaseous oxidizing agent (sol + gas), and solid fuel -
liquid oxidizing agent (sol + liq). In the combustion of sol + sol and sol + liq systems, the initial reagents were preliminarily mixed. In the first case these were pressed samples, in the second powders covered with liquid or suspensions. In the combustion of sol + gas systems, porous samples pressed from a powder of the fuel and placed in a medium of the gaseous oxidizing agent were used. The combustion process was carried out in constant-pressure bombs [10], widely known from the practice of investigation of the combustion of explosives, or in special reactors. The reaction was initiated with an igniting device [9]. The conditions of the experiments for various systems are cited in Table 1 and in Figs. 1 and 2.
We should note that carrying out a process in which liquid nitrogen participates in cryogenic hermetic reactors permits the synthesis to be conducted at high temperatures and pressures, created by the process itself, without the use of special techniques, which is a vital advantage of this method. In the experiments the rate of combustion was usually measured (Fig. 3) and an analysis was made of the chemical phase composition of the SHS product.
Branch of the Institute of Chemical Physics, Academy of Sciences of the USSR, Chernogolovka, Moscow Region. (Presented by Academician N.M. Emanuel, July 5, 1971.) Translated from Doklady Akademii Nauk SSSR, Vol. 204, N 2, pp. 366-369, May, 1972. Original article submitted May 7, 1971.
TABLE 1
| Reagents |
Dimensions of system, |
Pressure of gas,
atm |
Apparatus |
| fuel |
oxidizing agent |
mm |
inert |
reacting |
|
Solid (powders with dispersion 0.1—300 m)
Zr, Ti, Nb, Hf, Ta |
+ Mo, V |
Solid (powders with dispersion 0,1 m)
C, B, Si, S |
d = 5—30
l = 10—30 |
Argon
1—150 |
— |
Constant pressure bomb |
+ B, Al,
Mg, V |
Gaseous N2 |
d = 5—30
l = 10—30 |
— |
Nitrogen
0.5—1000 |
Constant pressure bomb |
| Liquid N2 |
d = 60
l = 140 |
Nitrogen, atmospheric |
Open reactor, immersed
in liquid nitrogen |
d = 30—60
l = 100—350 |
Nitrogen, initial
1—150,
final < 4000 |
Cryogenic hermetic reactors |
 |
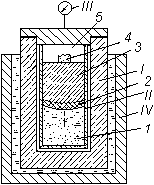 |
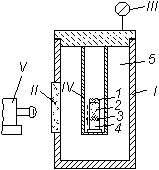
Fig. 1. Constant-pressure bomb.
1) Casing of bomb;
II) window for observation;
III) manometer;
IV) sample;
V) photorecorder of rate of combustion;
1) igniter;
2) combustion product;
3) reaction zone during combustion;
4) unignited portion of sample;
5) argon (or nitrogen);
¯) direction of propagation of combustion front.
|
Fig. 2. Cryogenic reactor.
I) Casing of reactor;
II) beaker;
III) manometer;
IV) Dewar flask;
1) mixture of metal-liquid nitrogen;
2) zone of conversion;
3) combustion products (nitride);
4) igniter;
5) excess nitrogen;
¯) direction of propagation of combustion front.
|
Systematic investigations of processes of SHS led to the following general results.
1. The process of combustion of the systems under consideration occurs in the condensed phase and is accompanied by right luminescence; the temperatures of combustion are 1500-3500°
C. The luminescent zone is propagated either in a smooth system (stationary, and sometimes even nonstationary), or in a pulsating system. The observed rates of propagation are 0.1-15 cm/sec. After passage of the combustion front the sample continues to luminesce for a time exceeding (sometimes substantially) the time of thermal relaxation (cooling). This is due to presence of two macrokinetic stages to the chemical reaction: the reaction in a narrow zone in the process of propagation of the combustion front and the reaction in the entire volume of the sample, heated by the combustion wave (complete combustion).
2. The combustion products are condensed at the temperature of combustion. The weight of the samples is either unchanged during combustion (systems sol + sol), or is increased (sol + gas) or is decreased (sol + liq) on account of partial evaporation of the volatile reagent. After combustion, the samples sometimes retain their original shape and size. The physical structure of the combustion products is different (unsintered powders, firmly sintered samples — porous or nonporous, solidified melt).
TABLE 2
| Compound |
Content of metal,
% by weight |
Content of nonmetal,
% by weight |
Structural type, symmetry group, and lattice periods,*
kX |
Microhardness, kg/mm2 |
Temperature of transition to the superconducting
state Tmax, K** |
| total |
free |
total |
free |
Titanium carbide |
80.0 |
<10-3 |
19,8 |
9 ·10-2 |
Cub. NaCl
a = 4,323 |
2900 |
— |
Zirconium carbide |
88,2 |
<10-3 |
11,4 |
<10-2 |
Cub. NaCl
a = 4,686 |
3040 |
— |
Hafnium carbide |
93,4 |
— |
6,3 |
<10-2 |
Cub. NaCl
a = 4,628 |
|
— |
Tantalum carbide |
93,6 |
— |
6,1 |
<10-2 |
Cub. NaCI
a = 4,453 |
— |
9,3 |
Niobium nitride |
86.9 |
<10-3 |
13,0 |
— |
Cub. NaCI
a = 4,385 |
1670 |
14,3 |
Zirconium nitride |
87,3 |
<10-3 |
12,6 |
— |
Cub. NaCI
a = 4,576 |
1570 |
9,6 |
Titanium nitride |
77,6 |
<10-3 |
21,4 |
— |
Cub. NaCl
a = 4,24 |
1800 |
— |
Hafnium nitride |
92,5 |
<10-3 |
7,2 |
— |
Cub. NaCI
a = 4,510 |
— |
6,6 |
Tantalum nitride [12] |
91,8 |
— |
7,5 |
— |
Cub. NaCI
a = 4,323 |
3200 |
— |
Boron nitride |
Â, 43,8 |
Â, 0,2 |
55,7 |
— |
Hexagonal
a = 3,331
c = 6,662 |
— |
— |
Zirconium diboride |
80,3 |
— |
21,0 |
3.10-1 |
Hexagonal
a = 3,161
c = 3,518 |
2350 |
— |
Titanium diboride |
68,6 |
— |
31,0 |
10-1 |
Hexagonal
a = 2,619
c = 3,230 |
3500 |
— |
Hafnium diboride |
89,0 |
— |
10,7 |
<10-2 |
Hexagonal
a = 3,134
c = 3,467 |
2890 |
— |
Molybdenum boride |
— |
— |
— |
— |
Tetragonal
a =3,120
c =16,932 |
— |
— |
* Data of V.M.Shekhtman.
** Data of A.G.Rabin'kin.
3. Combustion in the system sol + sol occurs with condensed initial, final, and possible intermediate products and belongs to the type of "nongaseous combustion" [11]. In the system sol + gas, the fuel and the oxidizing agent are not preliminarily mixed, but are macroscopically separated in space, since there is usually far less of the gaseous reagent in the pores of the sample than is needed for combustion. The gas is delivered to the combustion zone hydrodynamically, by filtration through the porous substance. Filtration occurs spontaneously on account of the pressure difference as a result of absorption of gas in the combustion front. By varying the conditions of access of the gas to the reaction zone, different systems of combustion can be set up (layered, surface). In the system sol + liq, two limiting systems are possible: combustion at constant pressure (open systems) and at constant volume (hermetic vessels with great completeness of loading).
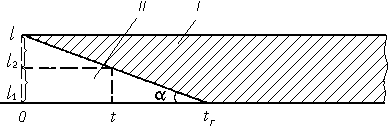 |
Fig. 3. Scheme of photorecording of the combustion process.
I, II) Illuminated and unilluminated portions of the photofilm, respectively;
l ) length of sample;
tc) time of combustion;
l1) unburned portion of sample;
l2) burned portion of sample at the moment of time
t. Rate of combustion u = tan a » l/tc.
|
4. The degree of conversion of the starting materials during combustion may differ and is determined both by thermodynamic (dissociation of the product) and by macro-kinetic (noncombustion) limitations. By regulating the process by varying the dispersion of the reagents, the size and density of the sample, heat removal from the surface, and temperature of combustion, completeness of the reaction can almost always be achieved (precisely, systems when there are practically no starting materials in the free state in the combustion products are established for purposes of synthesis).
5. In most cases the products of combustion are homogeneous and correspond to one chemical composition or another with different degrees of imperfection within the region of homogeneity. Both the composition and the degree of imperfection depend on the experimental conditions and can be controlled. As a rule, the products arc at equilibrium, with a well formed structure, although there are examples of the production of nonequilibrium and even amorphous compounds. The purity of the substances synthesized is no worse than the purity of the starting materials since there is no contamination during the reaction conducted by the SHS method, and completeness of conversion can be guaranteed.
The high quality of the products, simplicity of the apparatus formulation of the method, high rates of synthesis, absence of energy expenditures and theoretical size limitations, and the possibility of synthesis of metastable compounds and phases emphasize the practical advantages of the SHS method.
The authors are grateful to F.I. Dubovitskii for his valuable advice and aid in the organization of the work, to (G.N. Nechiporenko and B.M. Tarakanov for their consultation on the selection of the systems for investigation, to V.K. Enman and V.I. Ratnikov for their preparation of the apparatus, as well as to all the co-workers of the Laboratory of Macrokinetics of the Branch of the Institute of Chemical Physics, Academy of sciences of the USSR, who took part in the work.
LITERATURE CITED
- G.V. Samsonov and K.I. Portnoi, Alloys based on Refractory Compounds [in Russian], Moscow (1961).
- G.V. Samsonov et al., Boron, Its Compounds and Alloys [in Russian], Kiev (1960).
- R. Kiffer and F. Benezovskii, Hard Materials [in Russian], Moscow (1968).
- G.V. Samsonov, Nitrides [in Russian], Kiev (1969).
- A.E. Van Arkel and J.H. De Boer, Zs. Anorg. u. Allgem. Chem., 148, 345 (1925).
- A.E. Van Arkel, Physics, 4, 286 (1924).
- L.E. Cambell, J. Electrochem. Soc., 96, 318 (1949).
- A.K. Powel, J. Oxley, and J. Bloger, Jr., Eds., Precipitation from the Gas Phase [Russian translation], Moscow (1970).
- A.G. Merzhanov, V.M. Shkiro, and I.P. Borovinskaya, USSR Patent No. 255221, Byull. Izobr. No. 10 (1971).
- K.K. Andreev and A.F. Belyaev, The Theory of Explosives [in Russian], Moscow (1960).
- F.I. Maksimov, A.G. Merzhanov, and V.M. Shkiro, Phizika Goreniya i Vzryva, No. 4 (1965).
- A.G. Merzhanov, I.P. Borovinskaya, et al., USSR Patent No. 264365, 1969, Byull. Izobr., No. 9, (1970).


