O-4-01: Electroexplosion of Metastable Metal Powders with Stored Energy
G.V. Ivanov, V.G. Surkov
Energy that is 1.5-2.0 times higher than sublimation energy is input in metals in 1 m sec as wires are electroexploded. The metal overheated to 20,000 – 40,000 ° C broadens in shock waves in aerosol form and cools at a speed of 106 – 108 deg/sec. Aerosols deposited (the installation productivity is to tens kilograms per month) represent electroexplosion powders (EEP) of a size less than 100 nm. They are stable at storage on air in open jars. The powders possess some unusual physicochemical properties:
Their excessive stored energy is 2-5 times higher than metal melting heats. This energy is released in the heat waves mode at the temperatures much lower than melting temperatures (400 ° C for Al, Cu, 150 ° C for Ag, Sn, 120 ° C for In) [1]. Al EEP reacts with water similar to alkali metals at 50-70 ° C with the release of hot hydrogen [2]. In standard calorimetric bombs Al EEP reacts with nitrogen in SHS mode at the pressures of 20 atm. at a 100 % AlN yield. Nitrides not oxides are formed in mixtures with lead and barium nitrides at burning.
Aluminium boride is formed in mixtures with commercial
boron in SHS mode. Al EEP - amorphous boron pellets were put on an electrically
heated plate. The process begins at 730 ° C
and occurs at a speed of 5 mm/sec, at a self-sintering to 1,000 °
C. Brass is formed in SHS mode in mixtures of a commercial Zn powder and
Cu EEP, at the temperatures within 170-200 °
C and self-heating to 800 ° C.
References:
1. G.V. Ivanov, B. G Ivanov, V.P. Kuznetsov. Formation of heat waves in dispersed metal media at the metastable state relaxation. 1st All-Union Symposium on Macrokinetics and Gas Dynamics. Abstracts, Alma-Ata, 1984.
2. B. G Ivanov., S.N Leonov., G. L. Savinov et al. Burning
of ultrafine aluminium with gel-like water. Physics of Burning and Explosion,
1994, N 4, p. 167.
O-4-02: Near Net-Shaped Alkaline-Earth-Bearing Ceramics
K. H. Sandhage*
Dept. Materials Science & Engineering, The Ohio State University, Columbus, OH, USA
*on sabbatical at the Technische Universtitat Hamburg-Harburg;
E:Sandhage@tu-harburg.de
O-4-03: Influence of Anti-Combustibles Additive on the Inflammability
of Foam Polymer
Combustion Problems Institute, 172 Bagenbay Bat., Almaty,Kazakstan,
480012
Two ways of foam-polymers inflammability reduction have been shown as the most perspective. The first way consists in drawing fire-protective layer on the material surface . However this layer can be destroyed at further use of the product. The second way consists in addition in the foam-polymers the various chemical compounds, which will create some kind of obstacle to access the material surface oxidation as a result of thermal decomposition. The present paper were guided by the second direction. The additive for foam-polyuretan and foam-polysterol on the basis of urea-formaldehyde pitch (UFP) and diammonium phosphate (DAP) has been developed as anti-combustibles additive. These substances were added in polymers in frothing process in various percentage ratio. The advantages of these additives are cheapness, insignificant influence on frothing process and complete ecological safety. Solid UFP which has been used as the additive is a waste production and was not suitable for all industrial applications.
The tests of the synthesized material have been carried out on installations " Fire pipe " and " Oxygen index ". The obtained results have shown increase of the foam-polyuretan and foam-polysterol oxygen index more than twice. Now we may consider the received materials as not light igneous but hard igneous under the given inflammability characteristics (GOST 12.1.044-84). The found results are compared to the world analogues.
These days work on foam-polyuretan and foam-polysterol
production with proposed anti-combustibles additive is conducted in industrial
scale.
O-4-04: Microstructure and Mechanical Properties
of the SiO2-- Al2O3--Li2O--MgO--ZrO2--TiO2 Glass Ceramic
1 Departament of Materials Science, University of Guelma, BP 401, Algeria
2 GEMPPM,U.A. 341, Insa-69621 Villeurbanne, Lyon, France
The objective of this study is to investigate the two aspects:
O-4-05: Heat Explosion in a Heterogeneous Medium
1 Analyse Numé rique, Université Lyon, UMR 5585 CNRS 6922 Villeurbanne Crdex, France
2 Center for Research in Fire and Explosion Studies, University
of Central Lancashire, Preston, PR1 2HE, England
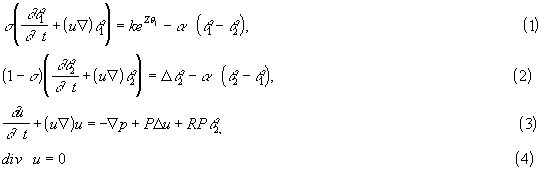
We consider the square geometry and assume that the side walls are heat isolated while the temperature at the upper and lower walls is equal to the ambient temperature. We study first the thermal model [1,2] of heat explosion (stationary solutions of (1) - (4) with zero velocity), relevant to condensed medium. Dependence on the coefficient of heat exchange, critical conditions of heat explosion, continuous branches of solutions were obtained as well as complete results on stability for a general positive and convex rate. These results can be applied to the case of heat explosion in a homogeneous medium. We study numerically the influence of natural convection on behavior of the two-phase system. If the Rayleigh number is sufficiently small, then the pure thermal regime [1,2] is stable and the medium is unmovable. If the Rayleigh number exceeds a critical value, then this solution losers its stability and convective structure appear. We study critical conditions of
convective instability and the interaction with convection.
Referenses:
2. C. Barillon, G.M. Makhviladze, V. Volpert, Stability of solutions for a model of heat explosion in
a two-phase medium. Submitted.
3. S. Goroshin, J.H.S. Lee, D.L. Frost, Combustion synthesis of ZnS in microgravity, 26h
International Symposium on Combustion, The Combustion Instotute, pp. 1651-1657, 1994.
4. A.G. Merzhanov, E.A. Shtessel, Free convection and thermal explosion in reactive systems,
Astronautica Acta, 1973, v.18, N 3, p.191-199.
O-4-06: Dynamics of thermal explosion in the post-induction period.
Institute of Structural Macrokinetics and Materials Science,
Russia Academy of Science, Chernogolovka, 142 432 Russia
Development of problems of self-propagating high-temperature synthesis (SHS) intensified interest to thermal explosion as a synthetic method in inorganic media. Due to conservation of hard mixture during all time of process one can investigate the dynamics of complete thermal explosion by means of the uniform macrokinetic mathematical model - model of gasless combustion.
In present report by computing simulation the dynamics of thermal explosion was considered in the post-induction period and the complete nonstationary analysis was realized. The depth of the volume of medium and the depth of conversion in frontal regime were investigated in the induction period.
Two mechanisms of the front propagation in the post-induction period were defined. We showed that SHS in conditions of thermal explosion ( samples in kiln or in the grafhite press-form ) has essential stage of self-propagating front. Therefore it is more correct to considere thermal explosion like way of the front synthesis initiation together with changing the initial temperature of mixture. The investigation were carried out for the wide diapason of changing parameters Fk, Bi, Td, Ze and for the different macrokonetic laws of chemical conversion.
Supported by Int’l. Sci. & Tech.Ctr. Grant 355-97.
O-4-07: Electrothermal Explosion and Gasless Combustion in
SHS Systems Containing a Melting Reagent
A.S. Shteinberg, K.V. Popov
Chernogolovka, 142432 Russia
In this connection, some data on SHS in layered samples
obtained by PVD (material widely applied in semiconductor industry) are
considered. Good agreement of theoretical
and experimental results testifies that joint application of kinetic method
of ETE and SHS research is an exceptionally promising direction for study
of layered samples with limiting layer thickness (down to 100 Å).
O-4-08: Modeling of Reactive Synthesis in Consolidated Blends of
B4C-Ti and BN-Ti
Department of Materials Engineering, Technion, Haifa 32000, Israel
O-4-09: Computer Simulation of Electrothermal Explosion in the Ni-Al System
Tomsk State University,Tomsk Branch of the Institute of Structural Macrokinetics
and Materials Science of Russian Academy of Science, Tomsk (Russia)
O-4-10: Effect of Shock Waves on SHS
Yu.A. Gordopolov, R.M. Shikhverdiev
Chernogolovka, 142432 Russia
O-4-11: Modeling of Solid-Phase Detonation
Department of Chemical EngineeringUniversity of Nebraska – Lincoln
Lincoln, NE 68588-0126, USA
O-4-12: Detonation Type Autowave in Phase and Chemical Transformation
Processes in Condensed Matter (Gasless Detonation)
1 Institut Nonlineaire de Nice, CNRS, Nice, France
2 Institute of Chemical Physics Researches RAS, Chernogolovka,
Russia
Our investigation shows that supersonic autowaves exit
in this gasless model (so as classical detonation). In the absence of a
dissipation factor (losses), continuum of travelling wave solutions is
found. In the presence of one( for example, diffusion) a steady state supersonic
wave solution is found.
O-4-13: Application of Explosive Shock Compacting to Functionally Graded
Materials Produced by SHS Reaction
R. Tomoshige1, H. Tanaka 1, A. Kato1, K. Imamura2, A. Chiba2
22-1 Ikeda, Kumamoto-shi, Kumamoto 860-0082, Japan.
2 Department of Mechanical Engineering and Materials Science, Faculty of Engineering, Kumamoto University, 2-39-1 Kurokami, Kumamoto-shi, Kumamoto 860-8555, Japan.
In this study, the hot-shock compaction technique was
also applied to the production of FGMs. The study shows it to be possible
to readily fabricate the dense TiC/Ti4Al2C2/TiAl
and TiB2/TiNi/Cu FGMs by the hot shock compaction method. Especially,
TiNi shape memory alloy was utilized in the TiB2/TiNi/Cu system
FGMs as an effective material, in order to reduce the thermal thermal stress
produced between both end materials by a reversible shape change of the
alloy, so-called pseudo-elastic effect. The FGMs were evaluated on their
microstructural characteristics and thermal properties. The particles in
the FGMs bonded strongly with each other. EDS analysis revealed that each
element was graded smoothly. Thermal shock tests revealed that the as-compacted
FGMs had high interlayer bonding strength, no exfoliation and no microcracks.
Thus, the SHS reaction had the potential to easily overcome the difficulties
in FGM fabrication
O-4-14: Optimum Choice of Three-Parameter Expression to Approximate
Dynamic Adiabatic Curves of Shs Systems and other Condensed Media
V.S.Trofimov
Analysis of shock and detonation adiabatic curves thermodynamic
equations was carried out at additional as sumption that was not used till
now at the present context. Namely, it was assumed that Grtneisen coefficient
monoto-nously approaches to its limit corresponding infinite increase in
pressure at constant volume. It turned out, that this assumption essentially
restricts type of expressions describing dynamic adiabatic curves of condensed
media. As a result it is succeed to describe uniformly equations of state
(dependence temperature of volume and pressure) and caloric equations of
state (dependence specific energy of volume and pressure) of various condensed
media. On this basis new simple methods of shock and detonation waves parameters
calculation in SHS-systems are proposed.
O-4-15: About Possibility of Gasless Detonation in Some Pyrotechnic Mixtures
E.A. Dobler, A.N. Gryadunov, S.A. Bostandjiyan, A.V. Utkin, V.E. Fortov
Institute of Chemical Physics Russia Academy of Science, Chernogolovka,
142 432 Russia
QPV<0 (1),
where QPV is the heat of the reaction at constant volume and pressure. This demand can be easily satisfied for reacting systems produces gases. But condition (1) holds and for some systems which are not producing gases systems. Most energy of the reaction in such systems are revealed in the form of the heat rather then in the form of the mechanical work and all work produced are performed by heat expansion of condensed products. So, detonation in such systems can be designated as gasless or heat detonation.
Satisfaction of (1) means that detonation can occur at some (may be infinite) diameters of reactive substance greater then some critical value. To provide detonation in the reasonable amount of substance the second demand must be satisfied: the time of reaction must be extremely small-a some microseconds (condition 2).
Current report devoted to the evaluation of possibility of the gasless detonation in metal - metal oxide pyrotechnic mixtures. Theoretical modelling of shock adiobates of reacting powders and detonation adiobates of products was used to test the possibility of the detonation regimes in some systems at different initial porosity’s. Necessary conditions for satisfying of (2) are discussed on the base of the experimental dates. As a result, the list of some perspective for gasless detonation systems with parameters of the possible detonation regime is presented.
The work was supported by Russian
Foundation for Basic Research (grants ¹ 96-03-32703a, ¹ 98-03-32201a, ¹
99-03-32262a) and the Russian Academy of Sciences (within the competition
of the young scientists projects in the field of chemistry, physical chemistry,
chemical physics and chemical engineering initiated by the RAS Presidium,
Project No.47, Decree 272, of July 13, 1998).
O-4-16: Liquid Flame in Gravitational Field
K.G. Shkadinsky1, G.V. Shkadinskaya1, B.J. Matkowsky2
2 Northwestern University, Evanston, IL USA
Multiple experiments on centrifuges, in the microgravity conditions and on earth showed existence (or absence) of separation in liquid products due to difference of the component densities. However the gravity separation begins after the solid matrix destruction (where components receive freedom of relative motion), i.e. in the preheat zone of the combustion front. Distribution of concentration and temperature is changed in the reaction zone. That influences on the front propagation velocity. In the present report the influence of the gravity fields on the propagation of liquid flame is investigated. We formulated a nonstationary mathematical model of liquid flame in the gravitational force fields. We carried out numericalsimulation and the analitical approximations of the model and compare to the experimental results. We studied the factors increasing (or decreasing) the combustion velocity with increase of the gravity acceleration. We showed that increase of the gravity acceleration can change the adiabatic temperature of combustion and the product structure. As for solid flame there are instability and nonuniqueness of the front propagation of liquid flame.
Supported in part by NASA Grant NAG3-1608, Int’l. Sci.
& Tech. Ctr. Grant 355-97.
J.H.S. Lee1, S. Goroshin1, R. Herring2
1McGill University, Montreal, Quebec Canada,E-mail: goroshin@mecheng.lan.mcgill.ca
2Canadian Space Agency, St-Hubert, Quebec, Canada,E-mail:
rodney.herring@space.gc.ca
O-4-18: Combined Effect of Magnetic Field and Gravity on SHS
Institute of Structural Macrokinetics and Materials Science,RAS,
Chernogolovka, 142432 Russia
Final products exhibited magnetization and coercive force
whose value were strong dependent on the orientation of attractive magnetic
field with respect to the direction of gravity. (i) samples have greater
magnetization and strong reduced values of coercive force compared to (ii)
and convenient SHS condition samples. The most obvious effect of magnetic
suspension took place when the combustion temperature was below the Curie
point of both the raw material and final product (Li0.5Fe2.5O4).
It is assumed that the observed increase in the magnetization and decrease
in coercivity are caused by difference in magnetic particles separation
phenomena in the gravity and magnetic field.
O-4-19: Gasless SHS in Microgravity
Institute o Structural Macrokinetics and Materials Science Russian Academy ofsciences
Chernogolovka, Moscow, 142432 Russia
In our experiments, we used spherical particles of AI cladded with Ni (the thickness of a Ni coating corresponded to a ratio of Ni/A1=1/1 for each cladded particle). Two types of samples were used: (a) cylindrical pellets of cladded powder and (b) loose powders with a different bulk density.
The pressed samples burnt in space and on the ground retained their cylindrical shape and size. According to the data of electron probe microanalysts and X-ray diffraction, the chemical and phase compositions are identical (NiAl). As follows from ESM analysis, the NiAl grains are larger in the space-produced material. For this material, the intercrystalline fracture is prevailing. Meanwhile, the ground-produced material exhibits marked transcrystalline fracture. Apparently, microgravity affects the crystallization process due to the absence of convection.
Burning of the loose powder at normal gravity proceeds without volume change. The space-produced product (NiAl) exhibits higher porosity and acquires the shape of the ampoule. Combustion velocity in the loose powder (under terrestrial condition) is about 1.5 cm/s. At microgravity, for the first time we observed the propagation of gasless SHS wave in the particle cloud in vacuum. The space-produced material exhibits a high-porosity skeleton (bound) structure.
The experimental results shown that space-produced pressed
samples of NiAl possess more perfect microstructure with larger crystal
grains. Gasless combustion of the vacuum particle clouds is the most interesting
result in the present work. A mechanism of this process must be studied.
High-porous continuous structure is formed due to change in the shape and
size of particles during combustion.
O-4-20: Effect of Microgravitation on Structure Formation of Refractory
V.N Sanin, V.I. Yukhvid, A.G. Merzhanov.
Institute of Structural Macrokinetics and Materials science of RAS
Chernogolovka, Moscow Region, 142432 RUSSIA., E-mail: svn@ism.ac.ru
In the work our attention was focused on the effect of
amount of a liquid phase in final and intermediate combustion products
on gravitational sensitivity SHS processes and structure of resultant products.
The detailed investigation of differences in course of SHS process and
formation of final products in space and terrestrial conditions was carried
out. The accuracy of the forecasting interpolated diagnostics was analyzed.
O-4-21:Self-Propagating High-Temperature Synthesis of Foam Cermet in Space
A.G. Merzhanov, V.A. Shcherbakov
O-4-22: Investigation of Effects of Electric Field on the Combustion Behavior in
the SHS Process by Use of the Heterogeneous Theory
Shizuoka University, Hamamatsu 432-8561, Japan
O-4-23: A Splitting Model for Interaction between Intense HF
Radiation and SHS Systems
Institute of Structural Macrokinetics and Materials Science,
Russian Academy of Sciences, Chernogolovka, 142432 Russia
The model involves (1) the constitutive relations of the
Maxwell equation for a green mixture (nonquasi-stationary approach), (2)
specially transformed Maxwell equations (suggested for the first time),
and (3) formal scheme for nonstationary energy dissipation in SHS systems.
The model was applied to a real SHS system taken as an example. In view
of complexity of the processes taking place in real SHS systems, predictions
of the model are only qualitative in their character. Instead of existing
"refined" modifications of the splitting technique, the model utilizes
a more rough approximation based on the Laplace–Karson operational calculus.
By its simplicity and physical clearness, the suggested technique was found
to be comparable with macrokinetic modeling. The model has two advantages
important for practical applications of SHS: (1) it is based on simple
mathematics (convenient for experimental checking of results) and applicable
to both direct and reverse problems of energy dissipation and (2) it provides
new convenient approaches to classification of the electrical properties
of SHS systems.
O-4-24: On the Field-Activated Combustion Synthesis of Titanium Aluminides
1Department of Chemical Engineering and Materials Science, University of California, Davis, CA 95616, USA.
2Dipartimento di Ingegneria Chimica e Materiali, University of Cagliari, Piazza D'Armi, 09123 Cagliari, Italy.
# On leave from (2)
The reaction mechanism of the field-activated combustion
synthesis (FACS) of the phase Ti3Al from elemental powders is
also investigated. Intermediates of the reaction leading to final product
are identified by performing X-ray diffraction analysis (XRD) and scanning
electron microscopy (SEM) of "quenched" samples obtained by turning off
the applied field during wave propagation. The combustion reaction is preceded
by the melting of aluminum, which then spreads over the titanium particles.
A reaction between liquid aluminum and titanium results in the formation
of a layer of TiAl3 which grows around the original Ti particles
until the aluminum disappears completely. Subsequently, the remaining (unreacted)
Ti interacts with TiAl3. At this stage, the particles consist
of Ti cores surrounded by a layer of Ti3Al which in turn is
surrounded by another layer of TiAl. This interaction further proceeds
leading to a shrinkage of the Ti cores, the gradual disappearance of the
TiAl layer, and the simultaneous increase of the Ti3Al
content. A single Ti3Al phase product is finally obtained.
O-4-25: Induction Field-Assisted SHS of AlN-SiC Solid Solutions
1 High-tech Research Center and Dept. of Materials Chemistry,
Faculty of Science and Technology, Ryukoku University, Ohtsu 520-21, Japan
2 Facility for Advanced Combustion Synthesis, Department of Chemical
Engineering and Materials Science, University of California, Davis,
CA 95612, USA
In this work we describe another modification of the field-activated
process: the use of an induced current. Samples covered with sheets of
carbon foil are placed inside an induction coil. Depending on the electrical
conductivity of the reactants, the induced current will be localized in
the foil or in both foil and the outer sample layer. Analogous to the case
of regular field-activation, two sources of energy can be present inside
the reaction zone: chemical and electrical. Moreover, the use of the induction
field-activation facilitates post-combustion annealing of the product.
In this paper we describe the results of an investigation on the induction
field-activated synthesis of AlN-SiC solid solutions and compare these
results with those obtained under normal field-activation.
Referenses:
1. Z. A. Munir, W. Lai, and K. Ewald, , U. S. Patent No. 5,380,409, January 10,
(1995); A. Feng, and Z. A. Munir, J. Appl. Phys.,1994, v. 76, p.1927..
2. H.Xue and Z.A.Munir, Ssripta Mater., 1996, v. 35,
p.979; J. Euro. Ceram. Soc.,1997,v. 101, p.1787
O-4-26: SHS in an External Magnetic Field (1.1T, 2T, 4T, 6T, 10T, 12T, 16T,
20T); Preparation and Characterization of Ferrites.
1 Department of Chemistry, University College London, 20 Gordon Street, London, UK WC1H 0AJ. (_ HYPERLINK mailto:i.p.parkin@ucl.ac.uk __i.p.parkin@ucl.ac.uk_)
2 Institute for Structural Macrokinetics and Materials Science RAS, Chernogolovka, 142432 Russia
3 Department of Physics, University College London, Gower Street, London, UK WC1H 6BT
4 Department of Physics, University of Nijmegen, Holland
O-4-27: Mathematical Modeling of Frontal Polymerization
Department of Engineering Sciences and Applied Mathematics, Northwestern University
Evanston, Illinois 60208-3125, U.S.A.
We develop mathematical models of FP and determine the
structure of the polymerization wave and its propagation velocity as well
as their dependence on the parameters of the problem. Our analytic results
are in good quantitative agreement with both numerical simulations of the
model and experimental data.
O-4-28: The Autowave Modes of Solid Phase Polymerization in
Two- and Three-Dimensional Composition Matrixes on the
Base of Fiberglass Fillers Appreting by Metal-Containing Monomers
V.V. Barelko, A.D. Pomogailo, G.I. Dzhardimalieva,
S.I. Evstratova, A.S. Rozenberg, B.M. Zuev
Chernogolovka, 142432, Russia
O-4-29: Organoelemental Synthesis in an SHS Mode
Institute of Structural Macrokinetics Russian Academy of Sciences, Chernogolovka, 142432, Russia, tel/fax:(095)9628040, E-mail: klim@ism.ac.ru
The important task of the subsequent researches is increasing of number of reactions classes, in which organic SHS is possible. In this connection, the attempts to organize autowave modes of reactions in organoelemental synthesis systems triphenylphospin/chloramine "B"and ferrozen/phtalic anhydrid/AlCl3 were undertaken.
The thermogravmetric research has shown existence of exothermic interaction near to melting point of low-melting reagent. In equimolar mixes of powders was a success to organize the SHS wave. The main schemes of chemical interaction are offered on the basis of the analysis data:
| ----------> (C6H5)3 PNSO2C6H5+NaCl+3H2O
|
(C6H5)3 P + C6H5SO2NNaCl·
3H2O---- |
|
| ----------> (C6H5)3PO+C6H5SO2NH2+NaCl+2H2O
(wave velocity u @ 4,0 mm/sec, max temperature Tm @ 1900C, conversion a = 100 %, output of a target product triphenyl-(phenylsulfonamide)phospinimide b@ 40 %);
AlCl3
Fe(C5H5)2 + C6H4C2O3
--------------------------> C5H5Fe C5H4COC6H4COOH
(u @ 1 mm/sec, Tm @ 190 C, a = 100 %, output of a target product o-carboxybenzoilferrozene b @ 16 %).
The results on study of the various factors influence
on the processes macrokinetics and structure of products, microstructure
of synthesized substances, date on DTA, NMR, X-ray analysis are discussed.
O-4-30: Frontal polymerization under conditions of weak convection
Institute of Continuous Media Mechanics, RAS, 1, Korolyov st., Perm,
614013, Russia;Tel. +7 (3422) 391 365; Fax: +7 (3422) 336 957
Referense:
1. Briskman V.A., Kostarev K.G., Lyubimova T.P. et al. Polymerization under different gravity conditions. Acta Astronautic, 1996, v.39, N 5, p.395-402.